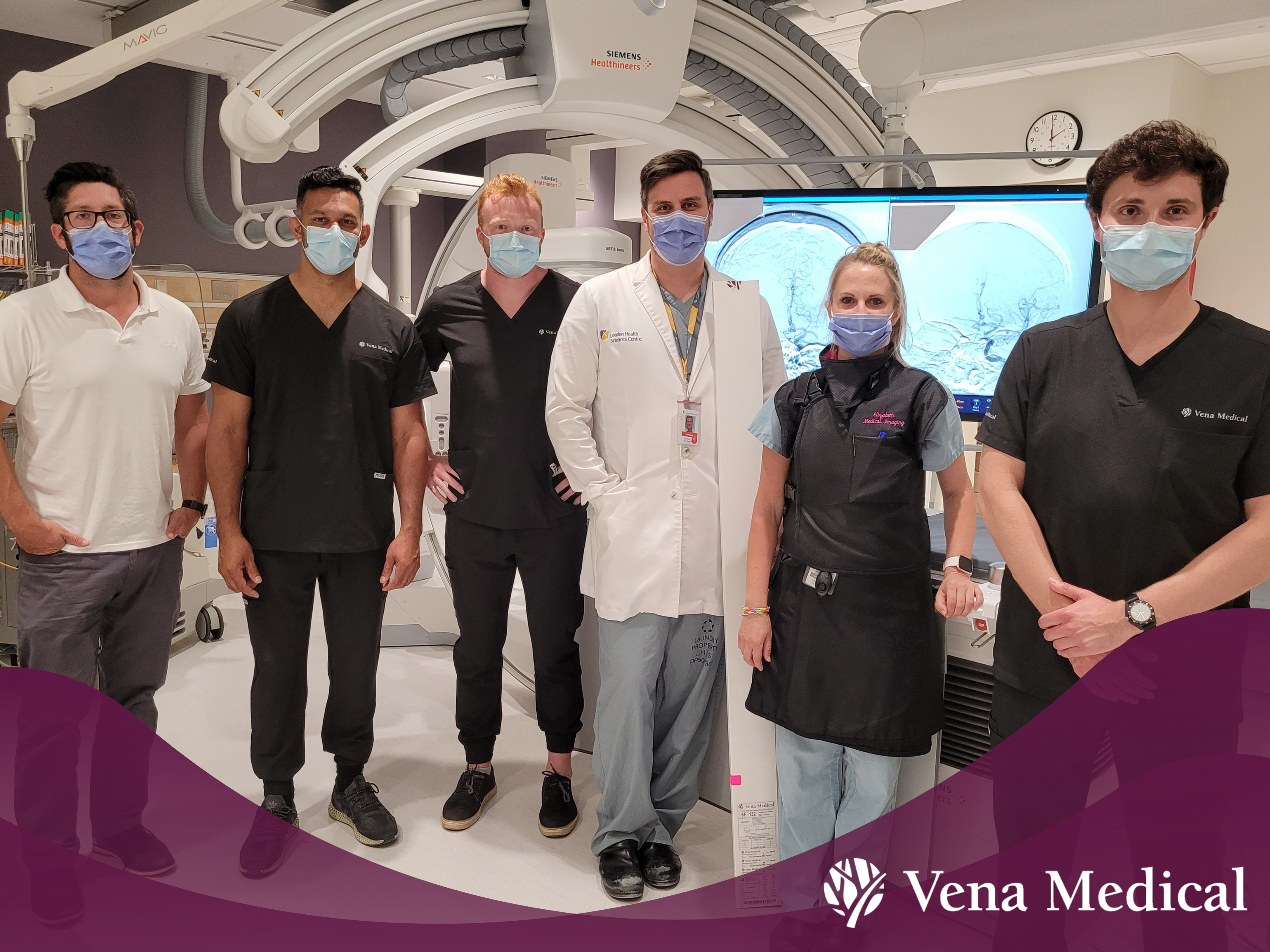
Vena Medical Celebrates First Procedure!
Kitchener medtech company treats first blood clot patients in the world with its new technology
A Kitchener-based medtech company, and current MIX resident is celebrating the treatment of the first patients in the world with its new cutting-edge technology. Vena Medical’s Balloon Distal Access Catheter (BDAC™) is a category-defining device, allowing physicians to remove a blood clot on the first try. Blood clots can be dangerous, and restoring blood flow as quickly as possible is critical. So far, the Vena BDAC™ has treated five patients across Canada, and could soon be in the hands of more world-class physicians at hospitals across the country.
First in-human procedure
The Vena BDAC™ was used for the first time on a patient during a 17-minute emergency procedure at London Health Sciences Centre University Hospital in June 2022. Vena Medical co-founder Michael Phillips was on-call to provide support to the hospital’s medical team on the use of the device during the procedure. Phillips recalls gripping his hands behind his back, describing the moment as a ‘white knuckle’ experience with ‘exciting’ results.
Before the procedure, the patient couldn’t use the right side of her body and had trouble speaking. Immediately after the procedure, the patient began using the right side of her body, and was able to speak and answer simple questions the following day. The patient is expected to make a full recovery.
How it works
Thrombectomy is the standard care of treatment for blood clots and several techniques are available for this procedure. However, with the Vena BDAC™, a blood clot can be pulled through to the device with no resistance, something that can’t be done with current devices on the market.
The Vena BDAC™ combines the balloon guide catheters and distal access catheters currently used in thrombectomy to remove clots from the brains of stroke victims. Combining these two devices allows the clinician to get the balloon much closer to the clot. So far, in all five procedures in which the device has been used on patients, the Vena BDAC™ got the clot out on the first try. The industry average for first-time blood clot removal is 35 per cent. Avoiding repeated attempts to remove blood clots results in better patient outcomes and reduced costs.
Using the Vena BDAC™ on a patient involves starting at the hip and entering the femoral artery. Physicians use a combination of needles and wires to slip the devices into that entry point, and use a sheath to hold the spot. The device is wired through the patient’s torso, up the aortic arch, and stops in the carotid artery in the patient’s neck. The Vena BDAC™ is then passed through the sheath to enter the patient’s brain, getting as close as possible to the blood clot. In the meantime, the medical team runs an x-ray and injects contrast to light up the blood vessels and locate the blockage. The balloon is inflated, allowing the BDAC™ to get a tight grip on the clot so it can be pulled out.
The future of blood clot treatment
Since the first procedure, other hospitals across Canada have started using the Kitchener-based medical technology company’s neurovascular device to provide ischemic stroke treatment. The first five patients in the world were treated with the device at London Health Sciences Centre University Hospital, The Ottawa Hospital and the University of Alberta Hospital.
London Health Sciences Centre and the University of Alberta Hospital bought the Vena BDAC™. The Ottawa Hospital procured the device through the VANISH trial (Evaluation of a New Balloon Distal Access Catheter for Stroke Thrombectomy), which is funded by the OBIO Early Adopter Health Network. This grant will help these hospitals evaluate the product and determine whether it can be used permanently for this procedure in their facilities.
Vena Medical is pursuing regulatory clearances, such as the U.S. Food and Drug Administration (FDA), to be able to treat patients across North America with its technology. In the meantime, more investigational studies are underway in Canada. Data is being collected during procedures, which will be shared with physicians to demonstrate how the Vena BDAC™ is outperforming the current gold standard. Vena Medical is looking forward to seeing its device in the hands of world-class doctors in more hospitals.
Celebrating medtech solutions in Southwestern Ontario
Founded by Michael Phillips and Phillip Cooper in 2016, Vena Medical started as a fourth-year design project out of the engineering program at the University of Waterloo. Vena’s flagship product is the world’s smallest camera, which allows physicians to see inside veins and arteries, to help them treat strokes. Phillips says the medtech company plans to have the Vena MicroAngioscope™ and Vena BDAC™ work together to treat patients.
Vena Medical obtained its Health Canada medical device licence earlier this year. Phillips says Vena Medical was able to obtain this licence with support from resources and mentorship opportunities provided through its partnership with Kitchener-based Medical Innovation Xchange (MIX). Founded in 2019, MIX is the first industry-led hub dedicated to helping medtech start-ups scale successfully in Canada.
A number of Canadian medtech companies often find a greater chance of success outside the country, where it can be easier to scale and commercialize. The support offered through resources, mentorships and facilities at MIX helps companies like Vena Medical keep their talent and technology close to home, and positions Southwestern Ontario as a world leader in innovative health solutions like the Vena BDAC™.

