MIX Profile: Ati Zarabadi, CEO of AiimSense
Every year, more than 15 million people suffer a stroke, with over half resulting in death or life-altering disability. While early diagnosis is the most critical factor in survival, current gold-standard diagnostic tools like MRI and computed tomography (CT) machines are immobile, expensive, and largely inaccessible outside major hospital centers.
One of the challenges for MRI and CT machines is the radiation and magnetic shielding required by the devices. But what if there was a way to put gold-standard diagnostic tools in a portable, low-cost package?
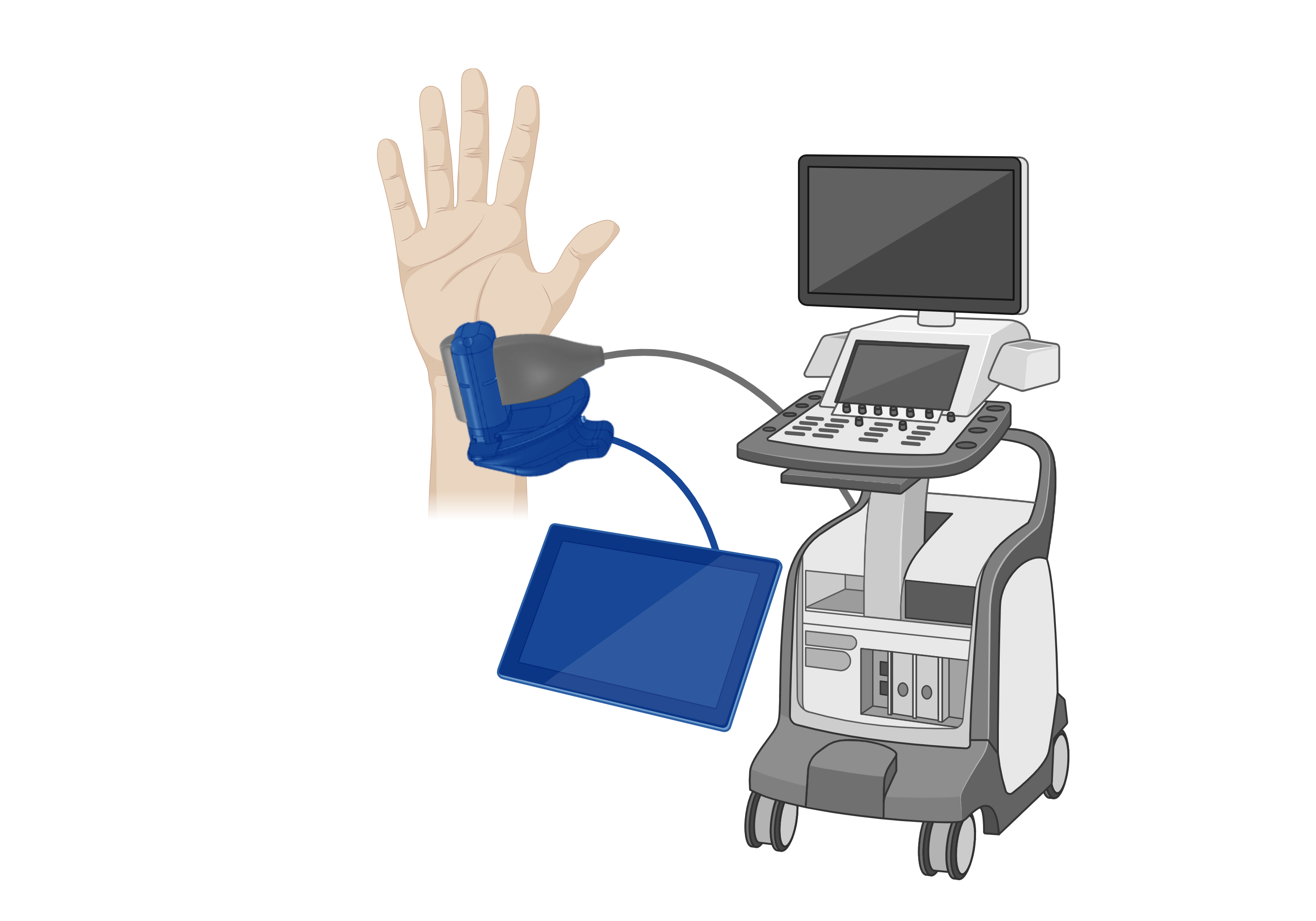
That’s the idea behind AiimSense, a first-of-its-kind microwave-based brain scanner that has the potential to improve stroke patient outcomes in big cities and rural locations.
From UWaterloo research to medtech reality
Ati Zarabadi was completing her PhD at the University of Waterloo when she met her co-founder, Mohammad Chavoshi. After exploring several clinical applications, the team identified stroke as an area with both urgent clinical need and a clear opportunity for technological differentiation.
“My background is in imaging where I specialized in spectroscopic imaging and molecular-level protein analysis. Mohammed brings deep expertise in microwave and RF technology, which is the core of our innovation,” Zarabadi says.
AiimSense leverages microwave imaging as an alternative modality to traditional MRI and CT. Historically, applying microwave technology to brain imaging was limited by hardware constraints and signal complexity. Recent advances in RF miniaturization and AI-driven signal processing have fundamentally changed that equation.
“The enabling technologies are now in place,” Zarabadi says. “What we are doing is integrating proven advances in hardware and AI into a purpose-built medical imaging system.”
Going from benchtop to bedside
The technical foundation of AiimSense rests on major breakthroughs in hardware miniaturization and advancements in AI algorithms. At the heart of their portable scanner is a Vector Network Analyzer (VNA), a device that measures the frequency response (magnitude and phase) of RF and microwave devices.
“In the 1970s, a VNA required an entire room of equipment to function. Today, it has been shrunk down to the size of a human palm,” she says. “You can imagine that it was not possible before, but with all these advances, it is becoming feasible.”
The journey to commercialization has been a methodical progression through increasingly complex validation stages. The team began with benchtop prototypes, using mannequins designed to mimic the dielectric properties of a human brain to prove they could detect a stroke. From there, they moved to preclinical animal studies, successfully monitoring the progression of ischemia in rodent models.
Now, AiimSense is entering its most critical phase—transitioning to human clinical trials. Through a partnership with the Calgary Stroke Program, the company is expanding its operations to Alberta to begin first-in-human testing.
Because microwave-based brain imaging represents a new category, AiimSense is pursuing a De Novo regulatory pathway, which allows innovative technologies to establish a new standard of care.
“This pathway provides a clear and structured route to market for novel devices,” Zarabadi says. “It aligns well with technologies that offer differentiated clinical value.”
The ecosystem behind the innovation
Developing a category-defining medical device requires technical expertise and the “been there, done that insights” from those who have navigated the complex business and regulatory landscape of medtech devices. For AiimSense, joining the Medical Innovation Xchange was a way to get both.
“What MIX helps with goes beyond the surface layer. They help identify the needs of a startup company,” she says. “This is especially important for companies like ours who are technical founders and need to get engaged with the business side of running a medtech startup,” Zarabadi says.
Part of that business journey involves securing the company’s intellectual property. AiimSense has approximately ten utility and design patents in progress, with a major milestone arriving this month.
“We recently got an allowance from the patent office that one of our core technology patents is going to be granted in January. That significantly strengthens our competitive position,” Zarabadi says.
Support from programs such as ElevateIP and Intellectual Property Ontario (IPON) has enabled the company to build a robust IP portfolio while maintaining capital efficiency.
A collective effort for brain health
Despite the long timelines and the high stakes of clinical trials, Zarabadi remains focused on the human impact of the technology. It’s a mission that hits close to home for the founders.
“My own father and my co-founder’s father both had a stroke, so we can see the value of developing this technology” she says.
While the medtech space is often defined by its competitive landscape, Zarabadi views the development of life-saving technology through a different lens.
“I always appreciate the work that our competitors are doing. We like to monitor and observe their progression, and we wish the best for them. It might look rational on the business side, but when it comes to bringing this life saving technology to the medical world, then I think we should all appreciate this collective effort,” she says.
Ultimately, for Zarabadi, the motivation comes from the fact that the technology is built for the people who need it most.
“With stroke, every second counts,” Zarabadi says. “A portable brain scanner has the potential to be a true game changer—for patients, clinicians, and healthcare systems worldwide.”